Heartworm Meds Reduce Cost for Low Income Families
- Research
- Open up Admission
- Published:
A statistical arroyo for evaluating the effectiveness of heartworm preventive drugs: what does 100% efficacy really hateful?
Parasites & Vectors volume 10, Article number:516 (2017) Cite this article
Abstract
Groundwork
Initial studies of heartworm preventive drugs all yielded an observed efficacy of 100% with a single dose, and based on these information the United states of america Food and Drug Administration (FDA) required all products to meet this standard for blessing. Those initial studies, however, were based on simply a few strains of parasites, and therefore were non representative of the full assortment of circulating biotypes. This event has come to lite in contempo years, where it has go common for studies to yield less than 100% efficacy. This has changed the mural for the testing of new products because heartworm efficacy studies lack the statistical power to conclude that finding zilch worms is unlike from finding a few worms.
Methods
To address this consequence, nosotros developed a novel statistical model, based on a hierarchical modeling and parametric bootstrap arroyo that provides new insights to appraise multiple sources of variability encountered in heartworm drug efficacy studies. Using the newly established metrics we performed both data simulations and analyzed bodily experimental data.
Results
Our results suggest that an important source of modeling variability arises from variability in the parasite establishment rate between dogs; not accounting for this can overestimate the efficacy in more than 40% of cases. We provide strong evidence that ZoeMo-2012 and JYD-34, which both were established from the aforementioned source dog, accept differing levels of susceptibility to moxidectin. In addition, we provide strong evidence that the differences in efficacy seen in two published studies using the MP3 strain were not due to randomness, and thus must be biological in nature.
Decision
Our results demonstrate how statistical modeling can improve the estimation of data from heartworm efficacy studies by providing a means to identify the true efficacy range based on the observed information. Chiefly, these new insights should assistance to inform regulators on how to move forrard in establishing new statistically and scientifically valid requirements for efficacy in the registration of new heartworm preventative products. Furthermore, our results provide stiff evidence that heartworm 'strains' tin can change their susceptibility phenotype over short periods of time, providing farther evidence that a wide diverseness of susceptibility phenotypes exists among naturally circulating biotypes of D. immitis.
Background
Initial studies of heartworm preventive products containing drugs of the macrocyclic lactone (ML) form all yielded an observed efficacy of 100% with a unmarried dose [one,two,3,4]. The experimental design used for these and other studies varied slightly, only the basic design was as follows. Dogs were infected with 30 to 50 infective L3 (iL3) stage larvae and and so assigned randomly to a non-treated control group and one or more than drug-treated groups. Grouping sizes varied among studies, but six to ten dogs per group were almost common. 30 days after inoculation with the iL3 larvae, the treated group(southward) was administered a single dose of the drug. Necropsy and consummate worm recoveries were done five to 7 months after infection to allow evolution to the adult parasite stage.
Considering all early studies yielded 100% efficacy, the Usa Food and Drug Administration (FDA) chose to crave that all drugs licensed for the prevention of heartworm infections encounter the 100% efficacy standard for approving. The first heartworm preventive product, which contained ivermectin (Heartgard-xxx®, Merial), received regulatory approval in 1987 [5], and this requirement for 100% efficacy has continued to this twenty-four hours. Those initial studies, however, were based almost entirely on a unmarried strain of Dirofilaria immitis (UGA-TRS) that had been isolated from a dog in the late 1960s, and so passaged in the laboratory approximately every three years to nearly the F10 generation until 2000 [6]. From the mid 1970s until the late 1990s, most heartworm preventive studies for all of the ML drugs submitted by animal health companies to the Center for Veterinarian Medicine (CVM)/FDA for product approving (ivermectin, milbemycin oxime, selamectin, moxidectin) were conducted using dissimilar generations of this same strain [vi]. Furthermore, it is estimated that no more than xv (and probably less) other strains from other laboratories were e'er used for those original studies, and most of those strains were used just in one case and never serially propagated in the laboratory [vi].
Moreover, early on publications demonstrating efficacy of these products consistently do not mention the name or geographic origin of the heartworm strain used in the study, nor practise they mention whether the strain was cycled in the laboratory, and if and then for how long. These issues were completely ignored; authors of these papers only state how many iL3 were administered. Thus it is non possible to know the exact number of strains used or anything else near them. Given this lack of documentation, it is incommunicable to know how many strains were used in the early studies of ML efficacy against heartworms. Withal, it is clear that (a) merely a small number of heartworm strains were ever tested, (b) geographic diversity and other factors that might affect the phenotypic and/or genotypic diversity of D. immitis biotypes circulating throughout the U.s. were not considered, (c) none of these strains underwent whatsoever formal or standardized process of characterization, and (d) the most commonly used strain was maintained in the laboratory for more than than thirty years and passaged to nigh the F10 generation [half-dozen].
In the above discussion, we use the term 'strain,' and here it is germane to discuss what is actually meant by the term. Isolates of D. immitis are about often referred to in the heartworm literature every bit 'strains,' and this nomenclature serves a purpose of convenience to distinguish i laboratory isolate from some other. The term strain implies genetic uniformity, notwithstanding, such as a genetic variant or subtype of an organism. Amid parasitic protozoans, the term strain is normally restricted to a homogeneous population possessing a fix of defined characters [7]. Given the high levels of genetic variety typical of nematodes, and reproduction by sexual ways, calling a parasitic nematode isolate a strain would rarely be scientifically accurate. However, D. immitis isolates are somewhat dissimilar than most other nematode species in that they undergo a fairly severe genetic clogging each fourth dimension they are passaged, because only about xv to 35 worms volition typically establish in a dog following an inoculation of 50 iL3. In addition, helminth parasites take a subdivided population structure as adult worms because they are confined to their definitive host and are only able to mate with worms co-inhabiting the aforementioned host [eight]. As a result many microfilariae circulating in the blood of an infected dog are partial or full siblings, and given that relatively few adult worms of each sex are present in a given dog, sibling matings in the F2 generation are probable, and increase in probability with each passage. This process greatly reduces genetic multifariousness in a heartworm 'strain' compared with what would exist with the establishment and passage of a strongylid gastrointestinal nematode, where thousands of worms typically constitute with each passage. Furthermore, this inherent bottlenecking that occurs each time a heartworm isolate is passaged, tin crusade the 'strain' to modify each time information technology is passaged, particularly in the offset few passages. The extent of these changes likely tin vary greatly from 'strain' to 'strain' depending on the level of genetic diversity present in the original field infection and the number of worms establishing each subsequent generation. The UGA-TRS strain used for the majority of product approving studies prior to 2000 [half dozen], would exist predicted to be highly bottlenecked and have very low genetic diversity every bit a issue of the repeated passage of this strain over thirty years.
In office to address this issue, in 2000 the FDA-CVM changed policy to require the use of newly acquired strains from different geographic regions in the US. Then in 2006, FDA-CVM unofficially refined this policy to crave that approval studies for new products use a strain that was isolated no more than 3 years earlier. These policy changes led to the isolation and maintenance of additional strains of D. immitis. Between 2000 and 2010, TRS Laboratories (Athens, GA) isolated seven additional strains that were used in the bulk of, if not all, heartworm efficacy studies performed in the US (past independent laboratories) over that time period [6]. These strains used would have undergone varying numbers of passages after being established in the laboratory, thus the degree of genetic and phenotypic change over time and betwixt studies likely varied for each strain. Furthermore, considering any given strain can but be used for 3 years, the strains used in product testing are constantly changing. Consequently, there is a loftier likelihood that any new production will be tested against a strain that represents a biotype that differs in genetic background and ML susceptibility from those strains that were tested previously. Finally, the limited numbers of strains bachelor for testing at any one fourth dimension almost assure that the strains used to test any new product volition non be representative of the full assortment of naturally circulating biotypes of D. immitis.
Defining efficacy and variability in efficacy – An result that needs reexamination
Efficacy can be defined every bit a quantitative measure of the effectiveness of a drug intended to produce a desired effect. With regard to anthelmintics, the expected or 'true' efficacy can be divers every bit the average efficacy of a given drug at the population level; significant beyond the entire population of dogs of varying breed, historic period, sex, weight, torso mass, health status, etc., that are infected with heartworms of differing biotypes at different infection intensities. Because one would have to test every dog in the population to determine the truthful efficacy, this is always an unknown value that is estimated. Alternatively, one can guess the truthful efficacy fairly accurately if both numbers of infected dogs tested and numbers of worms recovered from non-treated animals are very large (thousands of dogs of various signalments infected with tens of thousands of worms). Considering this is non a reasonable try, nosotros typically test a pocket-sized sample of dogs that are relatively uniform with regard to breed, age, weight, etc. This yields an observed efficacy that may or may not accurately represent the true efficacy. Clinical assessments of efficacy are then typically made based on the observed efficacy of a drug in a single study, and regulatory assessments are based on observed efficacies across several studies.
An observed efficacy of 100% ways there is no variability in the efficacy data of a given study. One time efficacy falls beneath 100%, nevertheless, variability in efficacy will always be present between animals in the same study and betwixt studies. This will be true whether or not the aforementioned or different strains are used, although if using different strains or biotypes variability is expected to be greater. In addition, in heartworm studies, the observed efficacy depends on the institution rate and this value is ever unknown for treated dogs. Consequently, every time a examination for efficacy is performed the outcome will be unlike, and the magnitude of the difference will depend on the amount of variability in the establishment charge per unit and the response to the treatment. Thus, the efficacy of a drug in any drug trial is not a fixed number, but lies within a set of possible values. This set of values can be described using a probability distribution whose parameters have both biological and statistical meaning [9]. To illustrate this point, in 1 of the early on studies with ivermectin, oral tablet doses of two.0 and 3.iii μg/kg administered thirty days subsequently infection were 100% constructive in preventing development to adults, but those same doses were only 97.two% and 98.ane% effective, respectively, in a 2d study [3]. In even so a third written report, an oral tablet dose of 2.0 μg/kg yielded only 83.3% efficacy [5]. None of these publications provide any information nearly which strain of D. immitis was used, and then one or more of these studies may have used a strain other than UGA-TRS. Regardless of whether these data represent testing done with a single strain, or with multiple strains, the ii.0 μg dose yielded efficacy results ranging from 83.3% to 100% illustrating the variability that is inherent in parasite efficacy studies. In the realm of heartworm preventive drugs, however, this outcome has since been virtually ignored because of the expectation of 100% efficacy at label dosages. Consequently, the upshot of D. immitis biotype/genetic variety and the potential differences in ML susceptibility of these various biotypes were non considered past manufacture and regulatory authorities. Admittedly, this consequence is non of import if at the characterization dose, differences in biotype/strain susceptibility are masked by an observed efficacy of 100% against all of them. The key question, however, which was never addressed, is "how many strains must be tested before one can reasonably conclude that all circulating biotypes of D. immitis would demonstrate 100% susceptibility to a unmarried characterization dose of ML drug, when tested using the accustomed/typical experimental model?"
A new phenotype appears
The second and third strains isolated by TRS Laboratories, 'Butch' and 'Missouri' (2000), demonstrated the aforementioned highly susceptible phenotype as the original TRS-UGA strain. In 2006, yet, the MP3 strain, just the fourth strain isolated by TRS, demonstrated a different phenotype. For the kickoff time, it was reported that a single characterization dose of both ivermectin and milbemycin oxime failed to attain 100% efficacy [10]. In that written report, one heartworm was institute in 1 domestic dog in each of the milbemycin oxime and ivermectin treatment groups. In an endeavour to come across the 100% efficacy requirement, additional studies were performed, using the formulated product (containing both spinosad and milbemycin oxime) at label dosages, to see if administering ii or 3 doses at monthly intervals would achieve 100% [11]. In those studies one treated domestic dog had one worm in one of the 2X treated groups (99.six% efficacy), and no worms were nowadays in the second 2X group or in the 3X treated group (Tabular array 1). Based on these (and possibly other) information, the FDA-CVM approved that product, but it was required that the label state that the drug should be administered "once monthly for at least three months later on exposure to mosquitoes." Information technology is noteworthy that the same product when tested confronting the Michigan strain (first isolated in 2007 by TRS Labs) yielded 100% efficacy with a single dose. Thus, a production that was just equally constructive as previously approved products required an boosted label statement solely because it was tested by adventure confronting a strain/biotype demonstrating a phenotype that was 'less susceptible' than those few strains used previously for production testing.
These data raised important questions about how efficacy of heartworm drugs is measured and interpreted. Information technology is important to observe here that the assessments described and so far are based on an observed efficacy without taking variability into account. As an alternative approach, it is preferable to analyze the data statistically, whereby variability is taken into account and the analyses provide interval estimates referred to as confidence intervals. With this information, one can determine with loftier probability whether the true (unknown) efficacy falls within this interval. Ane can then arrive at regulatory guidelines using the cease points of the confidence interval.
Prior to the studies with the MP3 strain referred to to a higher place, every new ML product approval study yielded 100% efficacy, thus interpretation of the information were straightforward. Statistical analysis of efficacy data seemed unimportant, demonstrated by the fact that in many, if not most of the early publications on efficacy of heartworm preventives, statistical analyses were not performed. In many of the more than recent publications statistical analyses are performed, and the most mutual approach is to first log transform the data, employ geometric means to calculate the efficacy, so perform analysis with a nonparametric test such as the Wilcoxon's Rank Sum Test [12]. Geometric ways are used because parasite data are usually over-dispersed and log transformation creates a less skewed distribution, and hence is less dominated by a pocket-size percentage of high values [13]. This produces a more appropriate estimate of central trend, and normalizes data enabling the assumption of normality required for parametric analyses. In fact, VICH international harmonization guidelines for anthelmintic efficacy recommend this approach [14]. Geometric means produce a bias, however, which causes differences in intensity to be exaggerated [xiii]. Moreover, a principal purpose of the log transformation is to enable the assumption of normality so that a parametric assay can exist performed. This is considering parametric analyses are preferred whenever possible because nonparametric tests have low power [15], yet nonparametric analyses are near common in the heartworm literature. This is non appropriate for several reasons, including the fact that the above-described methods do not take into account variability in the establishment rate. In addition, due to high toll and logistical considerations, heartworm efficacy studies tend to use relatively few dogs, and the loftier pathogenicity of D. immitis ways at that place are relatively few worms per dog. As a upshot, heartworm efficacy studies have innate low statistical power; thus parametric analyses are recommended. Furthermore, it is worth asking why parasitologists are still using an analysis method (Wilcoxon'south Rank Sum Test) published in 1945, before the existence of computers, when new powerful analyses exist that are much more appropriate for these types of information?
Now, allow u.s.a. return to the data reported by Snyder et al. [10, 11], where less than 100% efficacy was demonstrated against the MP3 strain with a single dose, both 100% and less than 100% efficacy were demonstrated with 2 doses administered 30 days apart, and 100% efficacy was demonstrated when three doses were administered at 30-day intervals (Table 1). Examination of these information leads to an important question: are these results actually biologically different from one another? Or were these different results more probable due to variability? Test of the 95% confidence interval (CI) suggests that variability was most likely responsible for the differences in the observed results between two versus three doses, since the 95% CI overlap. It is too possible that three doses are marginally more effective than ii doses; withal, these studies were insufficient in power to determine this. Based on these information and the results presented from the simulation (Tabular array ii), it seems adequately obvious that in a given heartworm efficacy report, there is insufficient belittling ability to say that one worm or zero worms in the treated group are unlike.
Methods and Results
Statistical issues related to efficacy
Analysis of information to evaluate the treatment efficacy is challenging, especially when the number of animals involved in the study is small. Worm count data tend to possess a variety of complicating characteristics such equally over-dispersion, disproportionate distribution, low counts, and excess zeros [9, 16]. These features in turn pb to high variability and hence, an authentic statistical assessment of efficacy is critical for a proper interpretation of the data. To statistically analyze such data, it is as well important to identify sources of variability and develop statistical methods that take into account underlying biological issues.
As a first step towards describing the efficacy of a treatment, 1 needs to have a precise definition of efficacy. In this manuscript, nosotros work with the following definition of efficacy (e):
$$ e=\frac{\mu_{ctl}-{\mu}_{tx}}{\mu_{ctl}} $$
(1)
Whereμ ctl is the population mean of worm counts in non-treated control dogs and μ tx is the population hateful of the worm counts in the drug-treated grouping. In the above formula, bothμ ctl and μ tx are population level quantities and are unknown. Information obtained from a given biological experiment(due south) are used to gauge the above parameters. Thus, while in experiments information technology may be the case that the estimates of μ tx equal zero, yielding an observed efficacy of 100%, this may be not the case in many experiments if this same experiment was repeated multiple times. The frequency with which a given outcome is observed depends on the truthful efficacy of the drug at the tested dose, and the amount of variability in the experimental organisation. Information that shows as a function of efficacy, how often 1 would see cipher, one, 2, or more than than two worms post treatment are shown in Tabular array two. From those data we discover that when the truthful efficacy is 99.95%, then 88% of the time we are unlikely to come across any worms post treatment when experimental groups incorporate 10 dogs. Thus one obtains a fake sense of 100% efficacy of a drug when the true efficacy is only 99.95%. In dissimilarity, when the true efficacy of a drug is 99%, then simply viii% of the fourth dimension would we expect to discover 100% efficacy. Furthermore, when efficacy is 99% there is a high likelihood that we might see whatever one of the following results: zero, one, two or more than ii worms (Tabular array 2).
Returning to sources of variability, an additional issue arises, equally it is impossible to determine what percentage of the inoculated iL3 remained viable in the treated grouping at the time the handling was administered. Under the study design described to a higher place, the non-treated command animals are used to approximate the percentage of the inoculated iL3 remaining viable in the treated group at the time of treatment. This is referred to as the institution rate. The establishment rate depends on an array of biological factors, including the dog's immune organisation, and hence is likely to change amidst dogs in a given heartworm study, every bit well as between studies. In addition, the strain used in a given study is very probable to impact the establishment charge per unit, although this has non been systematically examined. Thus, the estimate of the efficacy will vary betwixt dogs and between studies and hence it is of import to sympathize the estimated efficacy equally a distribution rather than every bit a unmarried number. Figure one illustrates this event, showing a histogram of the efficacy determined when performing simulations using published information for milbemycin oxime [17]. Note that when a biological experiment is performed, we can only observe a single fix of results. These results may be reflective of the true efficacy or may not be, depending on the amount of variability in the organisation and luck. By performing simulations (figurer experiments) using the observed data and an accordingly designed assay model, however, nosotros can determine both the probability of a given result and the range of expected results. Because the simulation is based on the observed biological data of a single experiment, the average and median issue of the simulation volition be shut to the observed result. Ane tin run across, notwithstanding, that a rather broad range of observed efficacies are possible; in this case the reported efficacy was 95.3%, but one could have gotten results that ranged from 89% to 99% (Fig. 1).
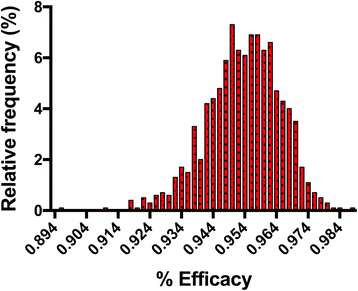
Efficacy distribution histogram of results from parametric bootstrap analysis of efficacy data for the milbemycin oxime group equally reported in Blagburn et al. [17]. Hateful and 95% confidence interval for efficacy are 95.3% (93.three, 97.1)
Returning to Table 2, the data presented were generated in a simulation where the parasite establishment rate was assumed to accept a mean of 50%, but allowing dog-to-dog establishment rate to vary. We selected l% because information technology is shut to the average seen across many heartworm studies in the published literature. Overall across published studies, hateful institution rates most commonly vary from approximately 30% to 70%, with individual dog institution rates varying from 0 to xc%. Such big differences volition profoundly bear upon the probability of observing diverse results. Thus, as variability in institution rates increases, and as true efficacy decreases (below the theoretical 100%), the amount of variability in the observed results will increment. Our simulation experiments prove that a variability of only five% in the establishment charge per unit can introduce a variability of upwardly to 13% in the observed efficacy. Nosotros also find that efficacy determinations that practise non business relationship for variability in institution rate can over-estimate the efficacy in more than 40% of cases. Similarly, non accounting for between-dog variability can overestimate the true efficacy in 27% of the cases.
Statistical modeling
We at present describe the statistical model used for analyzing information from the written report design described to a higher place. Permit the number of dogs in the control group and treated group be denoted past n. Permit Northward denote the number of worms inoculated into each dog, Y denote the number of worms recovered from a control domestic dog, and Ten the number of worms recovered from a treated domestic dog. These data when subscripted by k volition denote the respective values for each dog. As an example, Yone thousand will denote the number of worms recovered from the gthursday control canis familiaris while Tenyard will denote the number of worms recovered from the grandthursday treated domestic dog. Because the establishment rate changes between dogs due to a multifariousness of biological factors, we postulate that for the kthursday dog
$$ {\mathrm{Y}}_{\mathrm{k}}\sim {\mathrm{g}}_{\mathrm{k}}(.),\kern0.5em {\mathrm{Ten}}_{\mathrm{yard}}\sim {\mathrm{h}}_{\mathrm{m}}(.) $$
where gone thousand(.) and hk(.) are probability distributions with means μc,k and μt,k respectively. The subscript k in the means indicate that these means can change between dogs, and because it is difficult to place all the factors involved the term tin exist treated as being influenced by unobserved random variables. Nosotros call these latent furnishings. Some examples of these models are as follows:
- one.
Negative-Binomial /log normal model.
- 2.
Poisson /log-normal model
The population hateful response, which is the parameter of the worm count distribution, tin can likewise exist allowed to vary betwixt dogs. Specifically, one could model the worm count for a dog k in a treated or command group to exist Poisson with mean λk, where λk changes smoothly co-ordinate a distribution. This blazon of modeling is referred to every bit hierarchical modeling and the distribution describing the changes to λk is referred to as the random result or latent distribution. It describes variability in the responses due to hidden or unobserved biological factors.
While these models are useful to account for different sources of potential variation, inferences based on these models are difficult. There are no closed course expressions for the means, and variances are typically obtained using numerical methods. The conviction intervals can exist obtained from software such as SAS and R, just these analysis models are based on the assumption that the sample size is big. In our information sets, notwithstanding, we have only viii to 14 dogs per grouping, and this number cannot in general be considered as big.
An alternative approach to accost the effect of small sample size is via the apply of the bootstrap method [18] (Table 3). It is well known in the statistical literature that the parametric bootstrap method mimics the true inference (which would be the case when i knows the sampling distributions of the parameter estimates). For this reason, in the current paper we utilize the parametric bootstrap arroyo for inference concerning efficacies.
An important additional upshot that arises in the data sets for heartworm studies is that the establishment rate is not observable. The bootstrap algorithm described here facilitates incorporating the estimated institution rate and its variability in identifying the true efficacy. While parametric bootstrap is i approach, related Bayesian methods can also exist practical to obtain similar results.
Existent-life data illustrations on the usefulness and power of statistics in addressing biological questions
Illustration 1: ZoeMo-2012 vs JYD-34
We performed the analysis described in Tabular array 3 using SAS software (Version 9.iv), on efficacy data for two strains of D. immitis, JYD-34 and ZoeMo, both of which are derived from the same source dog. In this assay nosotros used Thousand = 1000 in the parametric bootstrap algorithm. Both JYD-34 and ZoeMo were established from blood samples nerveless from a heartworm microfilariae (MF) positive dog originally from Pittsfield, Illinois, but with little other known history. Thus the travel history, ML handling history, and age of the dog, likewise as the age of the heartworm infection are all unknown. JYD-34 was established first (by TRS Laboratories), equally a blood sample was used to infect mosquitoes on 13 July 2010 and TRS recipient dogs were infected with 50 iL3s on 29 July 2010. The JYD-34 isolate was validated in April 2011 with dogs testing positive for both MF and adult heartworm antigen. The JYD-34 strain was subsequently found to be resistant to ML drugs [19]. In contrast, the ZoeMo strain was established by Zoetis (Kalamazoo, MI) from a blood sample collected from the same source domestic dog, but approximately 17 months later on 4 December 2012. On 19 December 2012, ii dogs were each inoculated with 50 iL3 and these two dogs were positive for MF on 18 July 2013, validating passage of this strain. Interestingly, both of these strains were demonstrated to be ML-resistant, simply the observed efficacies for the two strains were quite different [20]. This so begs the question: Are the differences in observed efficacy a result of random variability, or is there a existent biological difference between the strains? To address this question nosotros analyzed data from efficacy trials using both strains where a single 3.0 μg/kg oral dose of moxidectin was administered 30 days following inoculation with 50 iL3. Furthermore, we analyzed data from JYD-34 comparing efficacy results of a single 3.0 μg/kg oral dose of moxidectin administered on twenty-four hours 30 with three sequent 3.0 μg/kg oral doses administered on days thirty, threescore, and xc.
ZoeMo-2012
The distribution of worm counts for the non-treated control and treated groups were determined to be Poisson based on Bayesian Information Criterion. Based on our assay the 95% CI for the efficacy of moxidectin was (78.32, 84.38) with an average efficacy of 81.63. This assay has the interpretation that for the observed number of worms in the moxidectin group, accounting for variability in the establishment rate, the interval (78.32, 84.38) captures the true efficacy 95% of the times. We refer to the above interval equally a provisional conviction interval. On the other manus, if the set of dogs in the data are considered to be a random sample from a population of dogs, then the CI for the efficacy of moxidectin is (74.21, 88.08) with an average efficacy of 81.47. We refer to this confidence interval equally a marginal conviction interval. From a regulatory perspective, we believe that the marginal CI is the more relevant value. Note that the average efficacy is nigh the same for both analyses, but taking all the sources of variability into account widens the CI.
JYD-34
We next plow to the JYD34 strain for a single dose of moxidectin. The distribution for the non-treated command and treated groups were determined to be Poisson based on the Bayesian Information Criterion. The conditional confidence interval for the efficacy was determined to be (seven.05, 24.77) with a mean efficacy of 16.09%. The marginal 95% CI for a single dose of moxidectin for the JYD-34 strain was determined to be (2.02, 28.05) with an average efficacy of xv.91%.
Nosotros next examine multiple doses of moxidectin. The distribution for the non-treated control group was adamant to exist Poisson and the distribution for the treated group was determined to be negative binomial based on the Bayesian Information Criterion. The conditional 95% CI for the efficacy was determined to be (29.87, 43.99) with a mean efficacy of 37.38%. The marginal 95% CI for the efficacy was determined to be (19.95, 53.15) with an average efficacy of 37.65%.
Comparisons of the data
ZoeMo-2012 vs. JYD-34
To compare the efficacies of a unmarried dose of moxidectin for ZoeMo-2012 and JYD-34, and a single versus multiple doses of moxidectin for JYD-34, we compared the distribution of the ratios of efficacies obtained using the parametric bootstrap model. If the differences in the observed efficacies were a consequence of randomness, then the 95% CI for the ratio of bootstrap efficacies should include one.0. In both comparisons, however, this was non seen. The 95% CI for the ratio of bootstrap efficacies for a single dose of moxidectin for ZoeMo-2012 and JYD-34 was (0.05, 0.35). These results establish that moxidectin was significantly more efficacious for ZoeMo-2012 than it was for JYD-34.
JYD-34, single vs multiple doses of moxidectin
The 95% CI for the ratio of bootstrap efficacies of a single dose versus multiple doses of moxidectin was (0.09, 0.88) with an average of 0.46 That is, the average efficacy of multiple doses of moxidectin is approximately 2.17 times the average efficacy of the single dose of moxidectin. This result shows that multiple dose of moxidectin were significantly more than efficacious than a single dose of moxidectin.
Interpretations of these analyses
ZoeMo-2012 vs. JYD-34
ZoeMo-2012 and JYD-34 are both derived from the same infected source dog, thus 1 would reasonably assume that these two strains would be very similar, and yield similar efficacy phenotypes. The only deviation between them is that ZoeMo-2012 was established 17 months after JYD-34. In both cases, the strain was established using 50 iL3. Looking at the information from two experiments, performed using the aforementioned protocol at the same laboratory, it appeared that Zoe-Mo-2012 was less resistant than JYD-34. The eye test, notwithstanding, is inadequate to make such a determination. Only by performing an appropriate analysis tin nosotros say whether those differences were most probable a outcome of random variability or of a biological crusade. Here, we demonstrate that those results are indeed most likely due to a real biological difference. Several possible explanations be, and one or more than of these may exist involved. The almost likely explanation would seem to exist that there was a mixed infection of both ML-susceptible and ML-resistant heartworms in the source dog, and that over the 17-calendar month menses at that place was a natural dice-off of more resistant worms than susceptible worms, thus making the overall infra-population of worms infecting the source dog less resistant overall. If this is true it may be that the resistant worms were acquired prior to the susceptible worms and were only dying of senescence preferentially due to their age. Alternatively, the resistant (or highly resistant) worms might take been less fit, and thus had a shorter life span than the susceptible (or less resistant) worms. An alternative caption is that the genetic bottlenecks inherent in the passage and establishment of heartworm strains, past randomness, caused the newly established derived strains to be somewhat genetically different. These possible explanations are simply an effort to explain an observation, and may or may not be a correct interpretation. Based on the analyses reported here, however, one matter we can say with confidence is that in that location was a true biological deviation in the infra-population of worms that became the strains JYD-34 and ZoeMo-2012.
JYD-34, single vs multiple doses of moxidectin
It is natural to expect that multiple doses of a drug are more effective than a single dose, and the observed efficacies in this case (37.vii% vs xv.nine%) lend credence to that expectation. Depending upon the number of dogs tested and the level of variability in the data, nonetheless, these differences may or may not be statistically significant. Here, our analysis demonstrates that this difference was non due to randomness, but rather to a real divergence in the efficacy of the treatment regimens.
Illustration 2: Efficacy of milbemycin oxime confronting the MP3 heartworm strain
Background to the outcome
2 studies published in early on 2011 received a great deal of attending, equally these were the first reports in which less than 100% efficacy was seen with a single dose of an ML-containing heartworm preventive drug, when administered at doses previously demonstrated to exist fully effective [x, 17]. Interestingly, although both studies demonstrated less than 100% efficacy, the efficacies they reported were vastly unlike. This was especially noteworthy at the time considering these ii studies were published in close time proximity to each other, the iL3 used for both studies were produced by the same lab (TRS Labs), under the same conditions, and the iL3 came from the same MP3 strain. Thus, ane would wait there to be essentially no differences in the biological science and genetics of the parasites used in the 2 studies. These disparate results led to a great deal of discussion and disagreement among the heartworm researcher community equally to whether these differences were due to randomness or due to a existent biological difference, and if biological, what the cause of the deviation might be. The disparities in the results of these studies, and the disagreement this provoked amidst experts in the field, provide an fantabulous opportunity to illustrate how statistics can exist used in a powerful mode to gain of import insights into biological questions.
Methods and results of the ii studies
In Snyder et al. [10], three groups of 14 dogs were each infected with l MP3 strain D. immitis iL3 and then treated 30 days subsequently with a label dose of either ivermectin or milbemycin oxime, or left untreated every bit controls. Five months mail service infection at necropsy, 13 of 14 dogs in the non-treated control grouping had adult heartworms, with a mean of 22.3 worms, whereas both the ivermectin and milbemycin oxime groups each had one domestic dog with ane worm, yielding a geometric mean efficacy of 99.8%. In Blagburn et al. [17], a similar but slightly unlike protocol was used. Here groups of 8 dogs were used, merely 100 rather than 50 MP3 strain iL3 were inoculated into each dog. Thus, the total numbers of iL3 administered per group of dogs were very similar for both studies (800 vs 700). Dogs were then treated 30 days later with a characterization dose of either ivermectin or milbemycin oxime, or left untreated equally controls (annotation that selamectin and moxidectin groups were also included in that study, but this give-and-take will not accost those data). In contrast to the results seen in the Snyder study, in the Blagburn study seven of viii treated dogs in both treated groups harbored worms at necropsy, with both the ivermectin and milbemycin oxime treated groups harboring a total of 23 worms each (range of 1–vi worms per dog). This yielded a geometric hateful efficacy of 95.half dozen%, and 95.iv% for the ivermectin and milbemycin oxime treated groups, respectively. Every bit a percentage of the number of iL3 inoculated into dogs in the two studies, the Blagburn study recovered 20 times as many adult worms at necropsy every bit the Snyder report. This begs the question: are the differences in the results of these studies uniform with an explanation of variability due to randomness, or is the magnitude of the difference better explained equally being due to a biological cause?
Modeling of the data and results of parametric bootstrap analysis
To accost this effect nosotros analyzed the raw information for the milbemycin oxime group from both studies with the parametric bootstrap algorithm equally described in Tabular array 3 using SAS software (Version 9.4), with M = 1000. The distribution of worm counts for the not-treated command groups in both studies were adamant to exist Poisson based on Bayesian Data Criterion. Based on our analysis, the 95% CI for the efficacy of milbemycin oxime were (99.2, 100) and (93.three, 97.1), with average efficacies of 99.8%, and 95.3% for the Snyder and Blagburn studies, respectively. The distributions of the bootstrap efficacies are shown in Figs. 1 and 2. These figures reinforce the concept that if the same experiment is repeated many times (here M = 1000), different results would be seen very frequently. The frequency with which each result occurred in our analysis is illustrated in these figures. Nosotros note that the confidence intervals for efficacy for the two studies practise non overlap, suggesting that the results are truly unlike. The near authentic way to address this, nevertheless, is not to compare the actual conviction intervals, simply rather to compare the 95% CI for the ratio of bootstrap efficacies for the two studies. This analysis yielded an interval of (0.936, 0.974); since this interval does not include 1.0, we can conclude that the efficacy seen in the Snyder report was significantly greater than that seen in the Blagburn study. Furthermore, nosotros tin can conclude that this difference is highly unlikely to have been caused past randomness, but rather must be due to a biological determinant. This assay cannot decide what biological factors were responsible; the only means to determine this would be to develop hypotheses and and so test those hypotheses in a biological system.
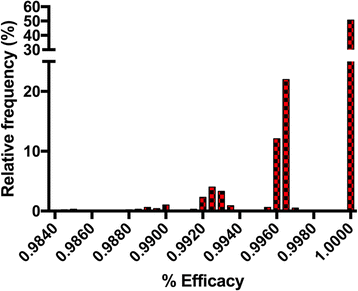
Efficacy distribution histogram of results from parametric bootstrap analysis of efficacy information for the milbemycin oxime group as reported in Snyder et al. [x]. Hateful and 95% confidence interval for efficacy are 99.8% (99.2, 100). Note that although one worm was seen in the biological experiment, the parametric bootstrap analysis yielded 100% efficacy more than l% of the fourth dimension
Estimation and insights from these analyses
In the aftermath of these reports, leaders in the heartworm community presented many different hypotheses to explain these differences. Throughout those discussions, information technology was assumed that both studies had used the same heartworm strain, referred to as MP3. But did they actually? Although these papers were accepted for publication inside 2 months of each other, the studies were performed about 2 years apart; the Snyder study in 2008 and the Blagburn study in 2010. Neither publication provides details of the origin of the parasites used other than referring to them equally from the MP3 strain. Recall from above that the MP3 strain was originally isolated and established in a dog at TRS Labs in 2006. The source of the MP3 parasites for the Snyder report was not the dog harboring the original infection, simply rather from a dog that had been infected via transplant with 10 pairs of adult worms in 2008. In contrast, the iL3 used in the Blagburn report originated from a canis familiaris infected via inoculation of 50 iL3 about 2 years later (JW McCall, personal communication, 2017). Although details of how many passages occurred through different dogs are not available, it seems clear that at least several passages occurred via both adult worm transplant and infection with L3.
As discussed earlier, every time a heartworm strain is passaged a genetic bottleneck occurs, and with such small numbers of worms establishing on each passage, randomness tin can loom large. These ii factors together, genetic bottlenecking and randomness, can take a major impact on the genetic diversity of the newly established version of the strain. To the recollection of the authors (RMK), this issue was never raised during the public discussions trying to observe an explanation for the big differences betwixt the studies. Rather, some proposed that randomness acquired the parasites to differ in the 2 studies, and others looked for methodological differences. Given the results of our analyses for these 2 studies, notwithstanding, and the analyses presented higher up for JYD-34 and ZoeMo-2012, information technology seems highly probable that a modify in the genetics (genotype) of the MP3 'strain' acquired a respective modify in the drug susceptibility phenotype of the MP3 'strain' used in the second written report. Although this may not explain the totality of the difference, information technology seems highly likely that it is the most prominent determinant. Although speculative, some other possible contributing factor may accept been the inoculation dose of iL3. As mentioned previously, the typical dose of iL3 administered in heartworm efficacy studies is 30 to 50 per domestic dog, and our expectations of efficacy are based on this experimental model. Only in the Blagburn study, 100 iL3 were used. If this was a contributing gene, it may suggest that efficacy decreases as parasite inoculum increases. This is an interesting question and deserves further investigation, especially since contempo studies suggest that the mechanism of activeness of ML drugs against D. immitis appears to involve the host allowed response [21, 22].
Give-and-take
In this paper nosotros provide a detailed overview of the bug involved in the measurement of efficacy for heartworm drugs, and demonstrate that use of a hierarchical modeling approach with parametric bootstrap tin provide elegant and statistical solutions to biological questions. Our results demonstrate how statistical modeling can improve the interpretation of data from heartworm efficacy studies by providing a means to identify the true efficacy range based on the observed information. Importantly, these new insights should help to inform regulators on how to movement forward in establishing new statistically and scientifically valid requirements for efficacy in the registration of new heartworm preventative products. Furthermore, our results provide stiff prove that heartworm 'strains' tin can change their susceptibility phenotype over short periods of time, providing further evidence that a broad variety of susceptibility phenotypes exist among naturally circulating biotypes of D. immitis.
For the outset 20 years of testing ML-based heartworm preventives, all studies yielded 100% efficacy (at characterization doses), and this became the standard required by regulatory authorities for blessing of new preventive products. Here, we make a case that insufficient numbers of heartworm strains/biotypes were used in testing to make such a conclusion, and we now know that "less susceptible" (MP3) and "resistant" biotypes (JYD-34) exist in the field, and are probably much more than common than generally appreciated. Furthermore, our results demonstrate that even for what is considered a heartworm 'strain,' that the susceptibility phenotype tin can change over a short catamenia of fourth dimension, and this is likely due to changes in the genetic makeup of the strain that occur with each passage.
Given this reality, it has become necessary to reevaluate the 100% efficacy standard and fifty-fifty reevaluate what 100% actually ways. Results presented here demonstrate that using appropriate statistical modeling and simulation, we can begin to empathize what it truly ways when we observe 100% efficacy in a pocket-sized heartworm efficacy trial, and too what it means when we see ane or two worms. Our analyses also demonstrate that differences in establishment rates can greatly impact the measurement of efficacy, and we know from a multitude of studies that institution rates vary widely both between dogs in a given study and between studies. Furthermore, the assumption of equal establishment rates in both the control and treated groups is inherent in the analysis and interpretation of all heartworm efficacy data published to date. Considering of inherent variability, however, this supposition is sure to be untrue, and thus this effect must be taken into account when developing an appropriate analysis model.
We also provide two real-life illustrations where nosotros used this statistical arroyo to accost biological questions then that firm scientific conclusions could be drawn from the data, as opposed to having unsubstantiated and subjective opinion determine the estimation. This same approach can be used on other data sets to address the core bug of the information, and can also be used to perform information simulations in club to better understand the problems at hand. Using this approach, it volition be possible to develop new regulatory guidelines that are based on both sound data and sound statistical principles, and that permit the estimation of the interval that contains the "true" efficacy. Results of our analyses demonstrate that this is a superior approach to basing clinical and/or regulatory decisions on an "observed" efficacy, which may or may not closely mirror the true efficacy. Knowing that a diversity of susceptibility phenotypes exist among circulating biotypes of heartworm, such an approach will permit us to move forward based on scientifically audio information, and improve how we evaluate the efficacy of heartworm drugs.
We accept demonstrated that observing 100% does not mean that a drug really is 100% efficacious against the strain it was tested against, let lonely all circulating biotypes. Nevertheless, our simulation (based on 10 dogs, 50 iL3, and a 50% institution rate) demonstrates that once efficacy falls below 99% it is likely that i or more worms volition exist seen, and once efficacy falls below 98% information technology is likely that ii or more worms will be seen. Given these data, ane could perform additional simulations and come upwardly with a lower 95% CI that would be required to ensure that the true efficacy of a drug meets at a minimum, a very high standard, without requiring the 100% standard, which is not verifiable.
Finally, nosotros find that most published heartworm efficacy studies do not provide the individual dog worm information, and only provide summary statistics for the entire group. This makes it impossible to re-analyze the data. Thus nosotros experience it is imperative that in the future, all published heartworm efficacy studies include the individual worm data; reviewers should require this. In addition, publications in the heartworm literature do not provide the full history of the strain being used and only state the strain proper name, as if the strain is a biologically static entity. Although this is a vast improvement over the early heartworm literature that most oftentimes did non fifty-fifty mention the strain name, information technology is nonetheless not plenty. Given the analyses presented here, it seems obvious that in every report or publication of a heartworm study that the total history of the strain used be provided in detail. Lastly, it would exist ideal if each heartworm 'strain' used in registration trials was divers by a prepare of genotypic markers, such equally microsatellites. This could and so serve equally a means to genetically fingerprint a strain, and potentially make up one's mind the degree of modify occurring as it goes through successive passages.
Conclusions
Powerful statistical models and data simulation provide a means to make accurate biological inferences on the observed efficacy. By linking biology and statistics, we are able to provide useful solutions to clinical questions, and ameliorate our ability to make evidence-based decisions.
Abbreviations
- CI:
-
Conviction interval
- iL3:
-
Infective third-stage larvae
- MF:
-
Microfilariae
- ML:
-
Macrocyclic lactones
References
-
Grieve RB, Frank GR, Stewart VA, Parsons JC, Belasco DL, Hepler DI. Chemoprophylactic furnishings of milbemycin oxime against larvae of Dirofilaria immitis during prepatent development. Am J Vet Res. 1991;52:2040–2.
-
Paul AJ, Todd KS, Acre KE, Plue RE, Wallace DH, French RA, Wallig MA. Efficacy of ivermectin chewable tablets and two new ivermectin tablet formulations against Dirofilaria immitis larvae in dogs. Am J Vet Res. 1991;52:1922–3.
-
Seward RL, Brokken ES, Plue RE. Ivermectin vs. heartworm - a status update. In proceedings of the heartworm symposium '86, New Orleans, Louisiana, march 21-23, 1986 (Otto GF ed.). American Heartworm Society. 1986:1–8.
-
Bater AK. Efficacy of oral milbemycin against naturally acquired heartworm infection in dogs. In Proceedings of the Heartworm Symposium '89, Charleston, South Carolina, March 17–19, 1989 (Otto GF, Jackson RF, Knight DH, Campbell WC, Courtney CH, Dillon R, Hite SC, Jackson RI, Levine BG, Lewis RE, Noyes JD eds). American Heartworm Gild, 1989.
-
Plue RE, Acre KE, Coleman MW, Currin ST, Ellis AJ, George LW. Efficacy and field safety of two new ivermectin formulations for the prevention of canine heartworm disease. In Proceedings of the Heartworm Symposium '89, Charleston, South Carolina, March 17–19, 1989 (Otto GF, Jackson RF, Knight DH, Campbell WC, Courtney CH, Dillon R, Hite SC, Jackson RI, Levine BG, Lewis RE, Noyes JD eds.). American Heartworm Guild, 1989.
-
McCall JW. Heartworm strains and testing preventive drugs. American Heartworm Society Bulletin. 2011;38:12–3.
-
Thompson RCA, Lymbery AJ. Intraspecific variation in parasites— what is a strain? Parasitol Today. 1990;6:345–8.
-
Churcher TS, Kaplan RM, Ardelli BF, Schwenkenbecher JM, Basáñez MG, Lammie PL. Mass treatment of parasitic disease: implications for the development and spread of anthelmintic resistance. In: Webber JT, editor. Antimicrobial resistance – across the breakpoint, bug in infectious diseases. Volume half dozen. Basel: Karger; 2010. p. 120–37.
-
Vidyashankar AN, Hanlon BM, Kaplan RM. Statistical and biological considerations in evaluating drug efficacy in equine strongyle parasites using fecal egg count information. Vet Parasito. 2012;185:45–56.
-
Snyder DE, Wiseman South, Cruthers LR, Slone RL. Ivermectin and milbemycin oxime in experimental adult heartworm (Dirofilaria immitis) infection of dogs. J Vet Intern Med. 2011;25:61–4.
-
Snyder DE, Wiseman S, Bowman DD, McCall JW, Reinemeyer CR. Cess of the effectiveness of a combination product of spinosad and milbemycin oxime on the prophylaxis of canine heartworm infection. Vet Parasitol. 2011;180:262–half dozen.
-
Wilcoxon F. Individual comparisons past ranking methods. Biom Bull. 1945;one:eighty–3.
-
Fulford AJC. Dispersion and bias: can we trust geometric means? Parasitol Today. 1994;ten:446–8.
-
Vercruysse J, Holdsworth P, Letonja T, Conder G, Hamamoto K, Okano One thousand, Rehbein S. International harmonisation of anthelmintic efficacy guidelines - (part 2). Vet Parasitol. 2002;103:277–97.
-
Tanizaki H. Power comparison of non-parametric tests: small-sample backdrop from Monte Carlo experiments. J Appl. Statistics. 1997;24:603–32.
-
Paterson S, Lello J. Mixed models: getting the best use of parasitological data. Trends in Parasitol. 2003;19:370–5.
-
Blagburn BL, Dillon AR, Arther RG, Butler JM, Newton JC. Comparative efficacy of four commercially available heartworm preventive products against the MP3 laboratory strain of Dirofilaria immitis. Vet Parasitol. 2011;176:189–94.
-
Efon B, Tibshirani B. An introduction to the bootstrap. New York: Chapman & Hall; 1993.
-
Blagburn BL, Arther RG, Dillon AR, Butler JM, Bowles JV, von Simson C, Zolynas R. Efficacy of iv commercially available heartworm preventive products against the JYD-34 laboratory strain of Dirofilaria immitis. Parasit Vectors. 2016;ix:191.
-
McTier TL, Vi RL, Pullins A, McCall JW, Rugg D, Maeder SJ, Wood DJ. Efficacy of oral moxidectin against susceptible and resistant heartworms in dogs. Parasit Vectors. 2017;10(Suppl 2): doi: 10.1186/s13071-017-2429-5.
-
Vatta AF, Dzimianski Thousand, Storey Be, Camus MS, Moorhead AR, Kaplan RM, Wolstenholme AJ. Ivermectin-dependent zipper of neutrophils and peripheral blood mononuclear cells to Dirofilaria immitis microfilariae in vitro. Vet Parasitol. 2014;206:38–42.
-
Wolstenholme AJ, Maclean MJ, Coates R, McCoy CJ, Reaves BJ. How do the macrocyclic lactones kill filarial nematode larvae? Invertebrate Neurosci. 2016;16:1–nine.
Funding
No funding was required for the writing of this manuscript. The article publication fee was funded by the American Heartworm Society.
Availability of data and materials
Not applicative.
About this supplement
This article has been published every bit part of Parasites and Vectors Book 10 Supplement 2, 2017: Proceedings of the 15th American Heartworm Lodge Triennial Symposium 2016. The full contents of the supplement are available online at https://parasitesandvectors.biomedcentral.com/articles/supplements/volume-10-supplement-2.
Author data
Affiliations
Contributions
All authors have read and canonical the final manuscript. RMK provided the clinical and biological perspectives and interpretations, ANV developed the analysis models and performed the information analysis and simulations, and PDJC assisted in the collection of data and literature used in developing this project.
Corresponding author
Ideals declarations
Ethics approving and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
In the past five years, RMK has received consultancy fees from Piedmont Pharmaceuticals and Merial addressing issues relating to efficacy of heartworm preventive products. The other authors take no competing interests relevant to the content of this newspaper.
Publisher'southward Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This commodity is distributed under the terms of the Artistic Eatables Attribution 4.0 International License (http://creativecommons.org/licenses/by/four.0/), which permits unrestricted utilise, distribution, and reproduction in whatever medium, provided yous give appropriate credit to the original author(south) and the source, provide a link to the Creative Eatables license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zip/1.0/) applies to the data made bachelor in this commodity, unless otherwise stated.
Reprints and Permissions
Well-nigh this article
Cite this article
Vidyashankar, A.Northward., Jimenez Castro, P.D. & Kaplan, R.M. A statistical approach for evaluating the effectiveness of heartworm preventive drugs: what does 100% efficacy really mean?. Parasites Vectors 10, 516 (2017). https://doi.org/ten.1186/s13071-017-2440-10
-
Published:
-
DOI : https://doi.org/10.1186/s13071-017-2440-10
Keywords
- Dirofilaria immitis
- Efficacy
- Macrocyclic lactone
- Canine heartworm
- Parametric bootstrap
- Statistical
Source: https://parasitesandvectors.biomedcentral.com/articles/10.1186/s13071-017-2440-x
0 Response to "Heartworm Meds Reduce Cost for Low Income Families"
Post a Comment